- Overview
- SARTATE
- SAR-bisPSMA
- SAR-Bombesin
- Scientific Presentations
SAR-Bombesin: A pan-cancer theranostic
SAR-Bombesin can deliver copper isotopes to GRPr expressing tumours and can be used to both diagnose and treat those tumours.
Bombesin is able to specifically bind GRPr which can be found in several cancers including:
- prostate cancer
- breast cancer
- ovarian cancer
- urinary cancer
- small cell lung cancers
- glioblastoma
- gastrointestinal stromal tumours.
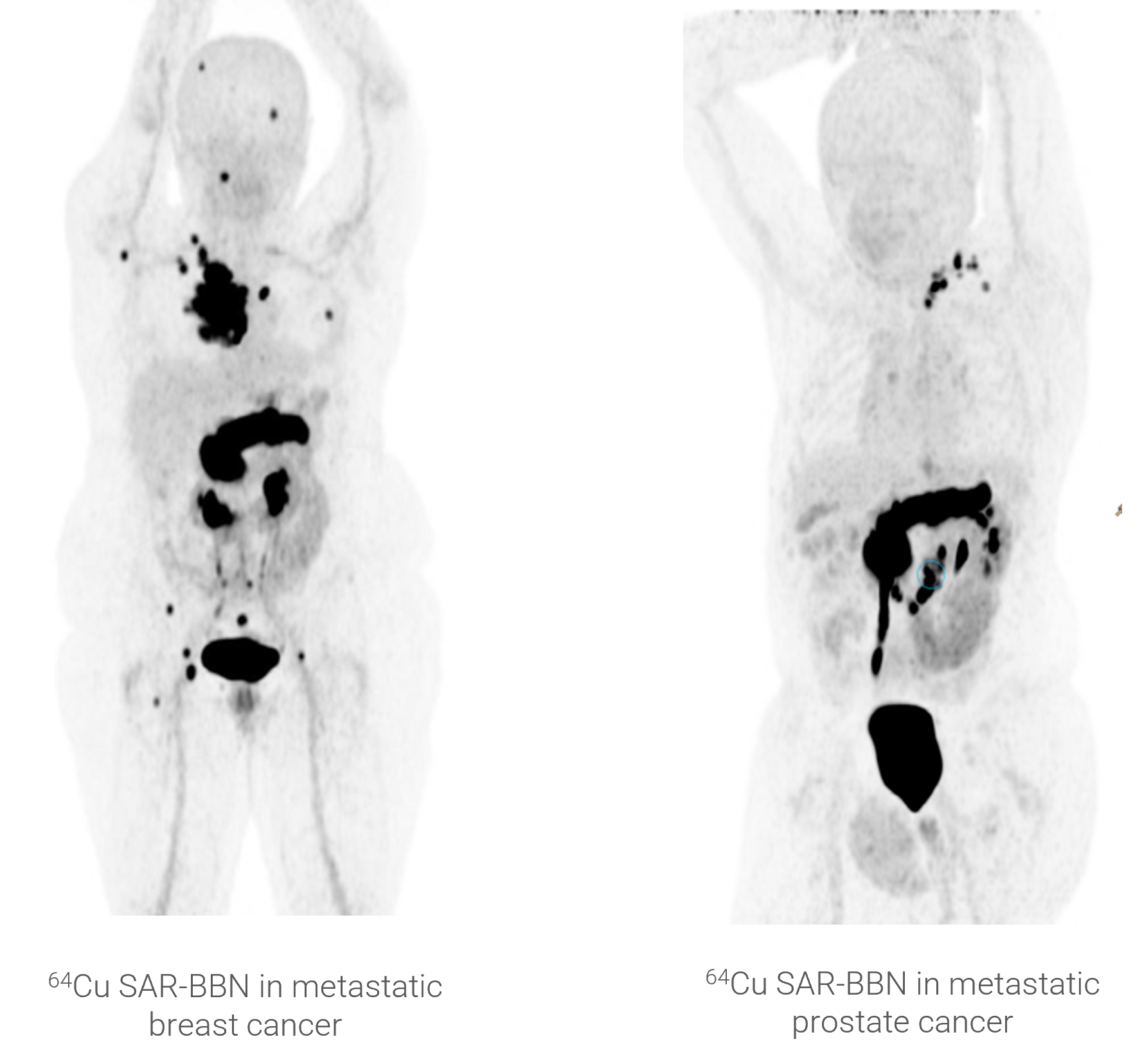
Clarity is initially developing SAR-Bombesin as a theranostic for breast cancer and prostate cancer. GRPr was shown to be expressed in 83% of estrogen receptor (ER) positive breast cancers and in 75-100% of prostate cancers.
Future opportunities for this product include imaging and treatment of other GRPr positive cancers. These indications open a large market opportunity for SAR-Bombesin due to high incidence or limited treatment options available to the patients with these types of cancers.
SAR-Bombesin Clinical Trial Overview
Study name | Products | Indication | Phase | Number of Patients | Location | Status | Reference |
64Cu SAR-Bombesin | Prostate diagnostic | II | 50 | USA | Recruiting | ||
64SAR-Bombesin/ 67SAR-Bombesin | Prostate therapy | I/IIa | USA | Open for recruitment | |||
BOP | 64Cu SAR-Bombesin | Prostate diagnostic | II | 30 | AUS | Recruitment complete | |
C-BOBCAT | 64Cu SAR-Bombesin | Breast diagnostic | I/II | 150 | AUS | Data published |
SAR-Bombesin and prostate cancer
Approximately 25% of metastatic castrate resistant prostate cancer (mCRPC) patients and 20% of biochemically recurrent (BCR) prostate cancer patients have low or no uptake of a PSMA-targeting tracer. These patients are therefore unlikely to show uptake of PSMA-targeted products, such as 68Ga-PSMA-11 for imaging or 177Lu-PSMA-617 for therapy, and currently have few radiopharmaceutical treatment options available to them. Given prostate cancer is one of the largest indications in oncology, there is a significant unmet medical need in this segment. The SAR-Bombesin product targets the GRP receptor found on prostate and many other cancers. As such, the SAR-Bombesin could offer valuable imaging and therapeutic options for not only PSMA negative patients, but also the large number of patients that have the target receptor on their cancers.
Active Clinical Trials


Copper-64 SAR-bisPSMA in biochemical recurrence of prostate cancer (SABRE)
SABRE is a Phase II Positron Emission Tomography (PET) imaging trial of participants with PSMA-negative biochemical recurrence (BCR) of prostate cancer following definitive therapy. It is a multi-centre, single arm, non-randomised, open-label trial of 64Cu-labelled SAR-Bombesin in 50 participants.
The primary objectives of the trial are to investigate the safety and tolerability of the product as well as its ability to correctly detect recurrence of prostate cancer.
Copper-67 SAR Bombesin in metastatic castrate resistant prostate cancer (COMBAT)
COMBAT is a dose escalation study with a cohort expansion. It is a US-based Phase I/IIa 64Cu/67Cu SAR-Bombesin theranostic trial for identification and treatment of mCRPC that is expressing the Gastrin-Releasing Peptide receptor (GRPr).
The aim for the study is to determine the safety and efficacy of 67Cu-SAR-Bombesin in participants with GRPr-expressing metastatic castrate resistant prostate cancer (mCRPC) in patients who are ineligible for therapy with 177Lu-PSMA-617.
Completed Clinical Trials
C-BOBCAT: 1 hour post 64Cu SAR-Bombesin administration in a breast cancer patient
64Cu-SAR-Bombesin in metastatic breast cancer (C-BOBCAT)
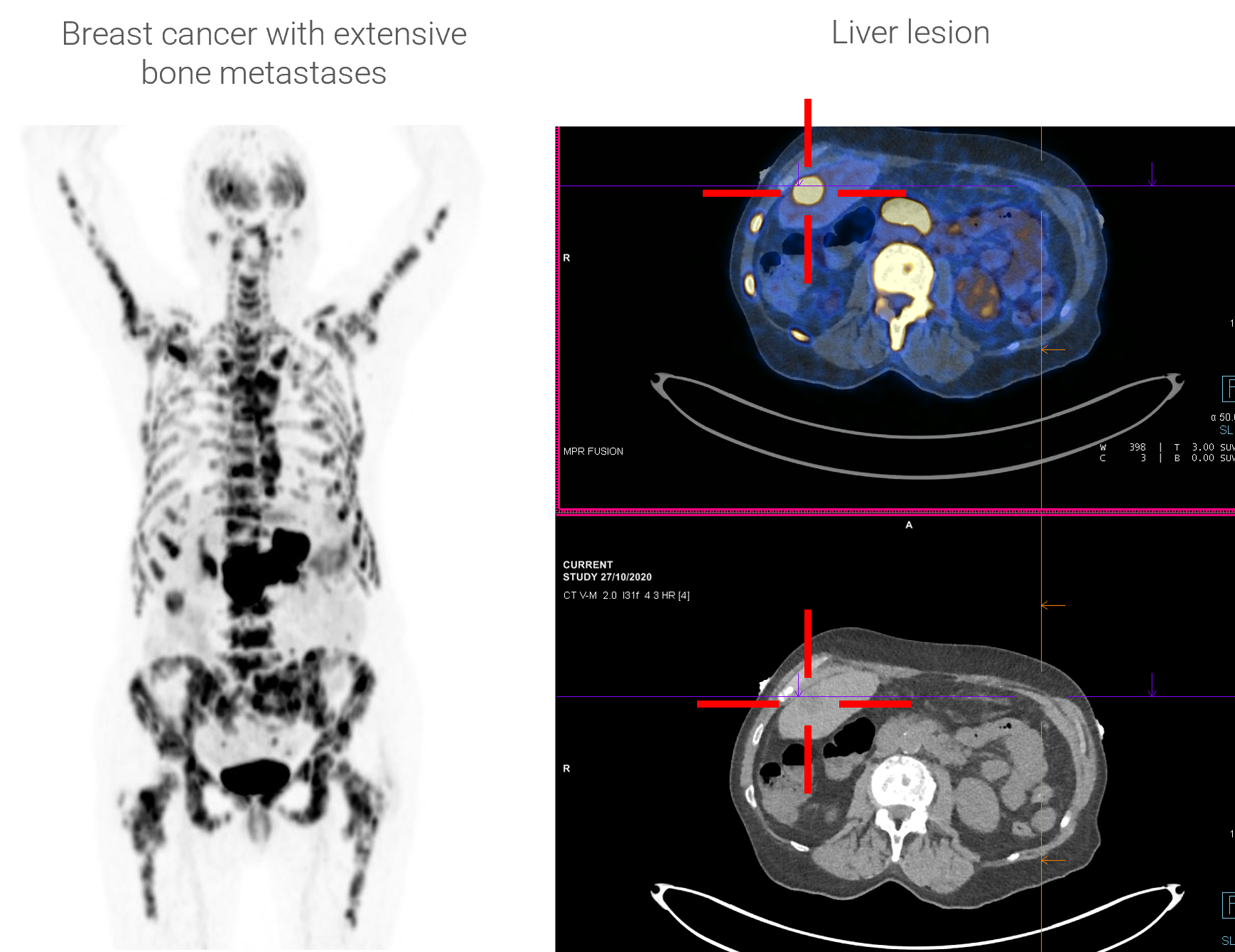
C-BOBCAT: Pilot trial assessment of the diagnostic value of 64Cu-SAR-Bombesin PET/CT imaging for staging of ER/PR + HER2- breast cancer patients with metastatic disease in comparison with conventional imaging (CT, bone scan and 18F-FDG PET/CT).
Trial status: Study objectives reached
Principal Investigator: A/Prof. Louise Emmett.
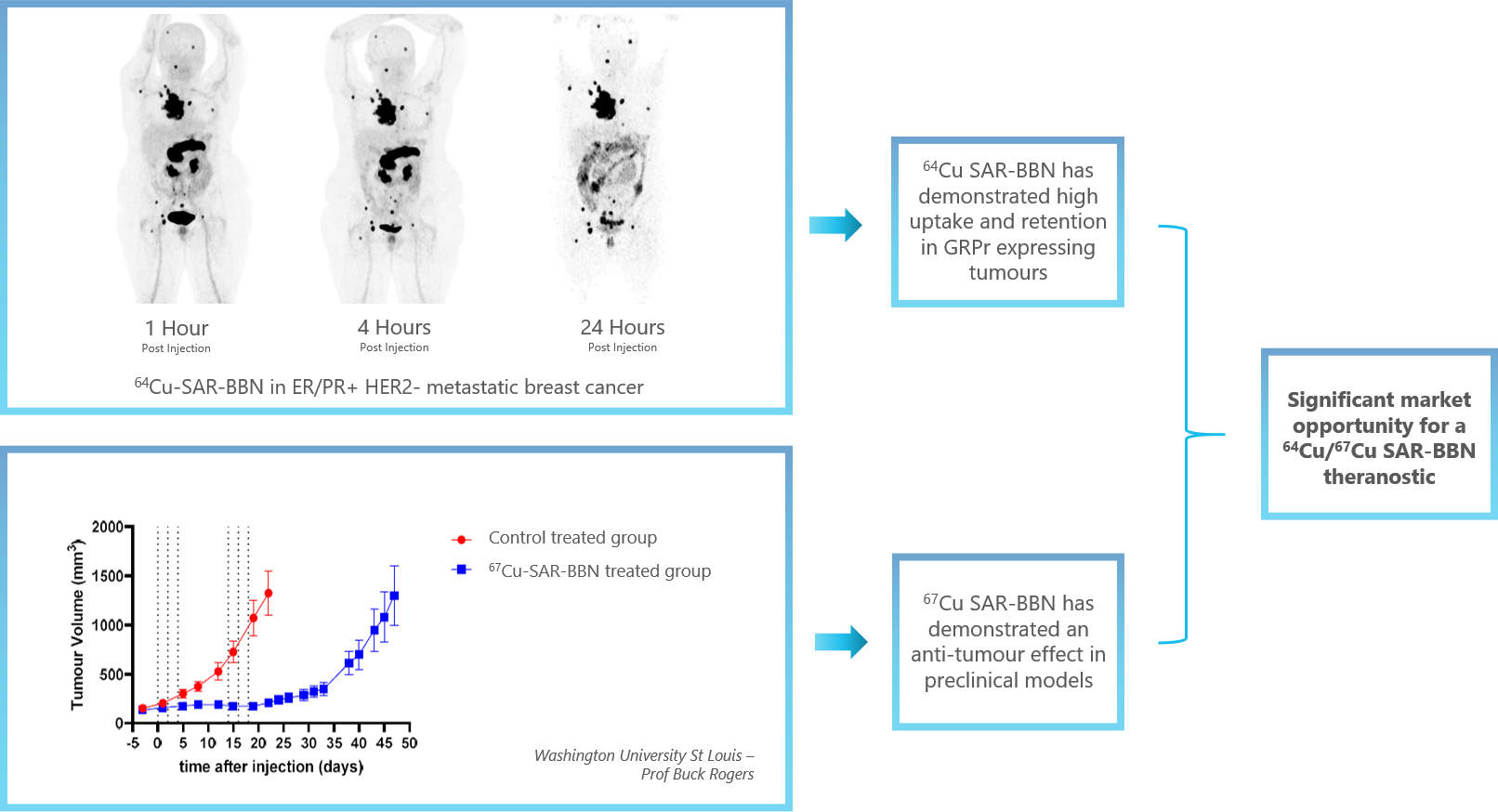
- The diagnostic program with 64Cu SAR-Bombesin generated evidence of the utility and potential superiority in some patient subgroups compared to conventional imaging (e.g. 99mTc bone scan, 18F FDG).
- The high uptake and strong product retention visualised by PET imaging of patients at 1, 3 and 24 hours after product administration suggest significant potential for therapy applications with 67Cu SAR-Bombesin.
- The human clinical data from the trial is used for Investigational New Drug (IND) Application filings with the US Food and Drug Administration (FDA).
- Clinical trials for SAR-Bombesin in the US expected to commence in 2022.
SAR-Bombesin Theranostic Opportunities