SARTATE combines the well characterised peptide octreotate, which has been in many thousands of patients to date, with Clarity’s proprietary SAR Technology and the isotopes of copper.
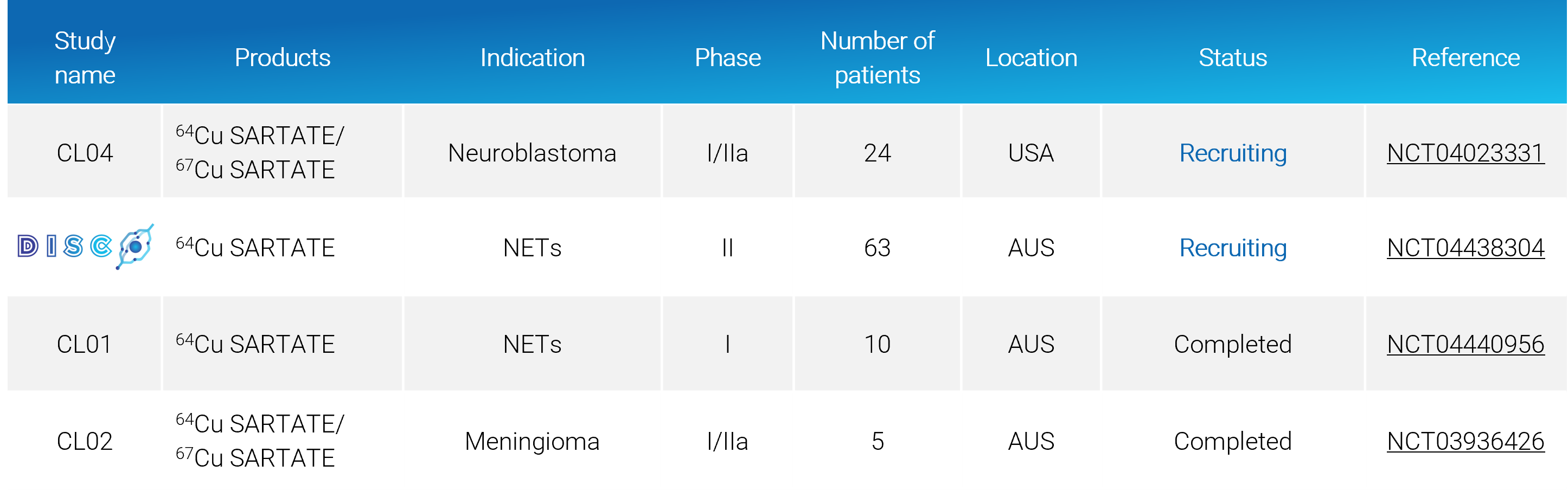
Study name | Products | Indication | Phase | Number of Patients | Location | Status | Reference | ||||||||||||||||||||||||
CL04 | 64Cu SARTATE/ 67Cu SARTATE Neuroblastoma I/IIa 24 USA Recruiting  64Cu SARTATE NETs II 45 AUS Closed recruitment CL01 64Cu SARTATE NETs I 10 AUS Completed CL02 64Cu SARTATE/ 67Cu SARTATE Meningioma I/IIa 5 AUS Completed |
Active SARTATE Clinical Trials
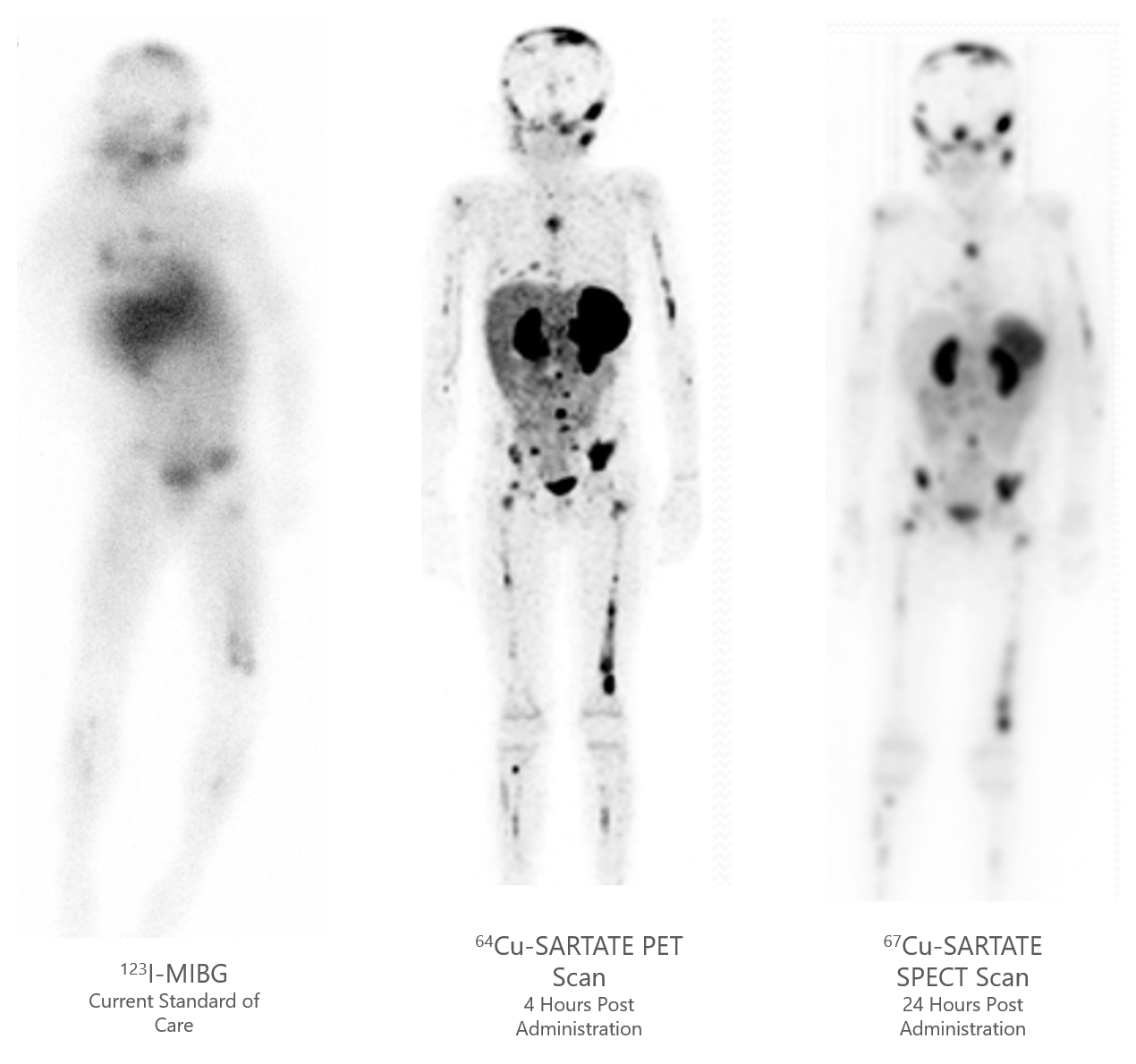
67Cu-SARTATE Peptide Receptor Radionuclide Therapy (PRRT) Administered to Paediatric Patients With High-Risk Neuroblastoma (CL04)
Reference: NCT04023331
Trial status: Recruiting
Multicentre, dose-escalation/cohort expansion study being conducted in the USA.
Clinical sites
- Memorial Sloan Kettering Cancer Centre
- Cincinnati Children’s Hospital Medical Centre
- Medical University of South Carolina
- University of Texas Southwestern Medical Centre
- University of Wisconsin
About neuroblastoma
Neuroblastoma is one of the most aggressive childhood cancers. Each year, there are around 800 new cases of neuroblastoma registered in the US (for more information click here). It is the most common cancer to be diagnosed in the first year of life and accounts for around 15% of paediatric cancer mortality. Around 85% of neuroblastomas express SSTR2.
Neuroblastoma is a rare paediatric disease and Clarity has received two Rare Pediatric Disease Designations (RPDDs) from the US Food and Drug Administration (FDA) for 67Cu SARTATE and 64Cu SARTATE
67Cu SARTATE and 64Cu SARTATE have been granted FDA Orphan Drug Designation (ODD) status for the treatment and clinical management of neuroblastoma.


A Diagnostic Imaging Study of 64Cu-SARTATE Using Positron Emission Tomography (PET) on Patients With Known or Suspected NETs (CLS07)
Reference: NCT04438304
Trial status: Recruitment closed
The purpose of this study is to assess the performance of imaging agent 64Cu SARTATE in participants with known or suspected Gastroenteropancreatic (GEP) NETs as a potential new way to help diagnose this type of cancer.
Clinical sites
- Royal North Shore Hospital
- Peter MacCallum Cancer Centre
- Royal Adelaide Hospital
- Nepean Hospital
About NETs
NETs, also known as well-differentiated neuroendocrine neoplasms or carcinoids, are a heterogeneous group of malignant transformations of cells of the diffuse neuroendocrine system. The most common site of primary NETs is the gastrointestinal tract (GI) (about 60% of all cases), followed by the bronchopulmonary tree (27%). Less frequent sites are the pancreas, biliary tract, liver, ovaries and testes (for more information click here).
The annual incidence of NETs is around 7 cases per 100,000.
A delay in diagnosis or misdiagnosis is common, such that most NET patients have metastatic disease by the time a diagnosis is confirmed. About 30-75% of NETs patients have distant metastases at the time of diagnosis according to the US and European cancer registries.
SARTATE Regulatory Milestones

Clarity has reached a number of regulatory milestones and approvals from the US FDA for the development of SARTATE, including the award of two RPDDs which may allow the company to access two Priority Review Vouchers (PRVs).
PRVs can be used for priority review of a subsequent drug or sold / transferred for use by another company on an unrelated drug and currently trade for ~ USD 100 million.
64Cu-SARTATE first-in-human diagnostic trial in NETs (CL01)
Reference: NCT 04440956
Site: Peter MacCallum Cancer Centre,
Principal Investigator: Prof Rod Hicks
In comparison to 68Ga-DOTATATE scans, 64Cu-SARTATE imaging in participants with NETs was equivalent at 1 hour, and superior at 4 hours, allowing detection of additional tumours at later time points.
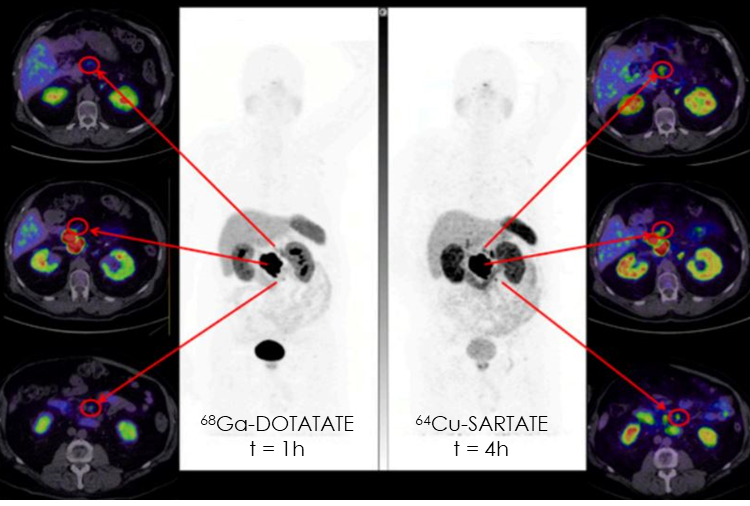
Superior lesion detection at 4 h with 64Cu-SARTATE (right) in the same patient compared to 68Ga-DOTATATE images at 1 h (left) in the same patient
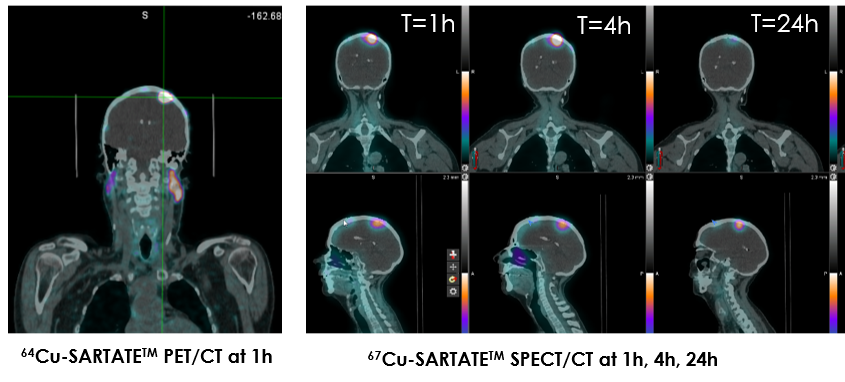
67Cu-SARTATE first-in-human theranostic trial in meningioma (CL02)
PRRT administered with 67Cu-SARTATE to participants with meningioma: A single-centre, open-label, non-randomised, Phase I-IIa theranostic clinical trial
Reference: NCT03936426
- SARTATE targets the tumour to the same extent when using either 64Cu-SARTATE (PET/CT scan) or 67Cu-SARTATE (SPECT/CT scan).
- The trial data demonstrated 67Cu-SARTATE (SPECT/CT scan) was generally safe and well-tolerated in adult cancer patients.